-
Products
-
- Featured Products
- Supraflex Cruz
-
- Professionals
- Patients & Caregivers
- About SMT
Main navigation
Key Features
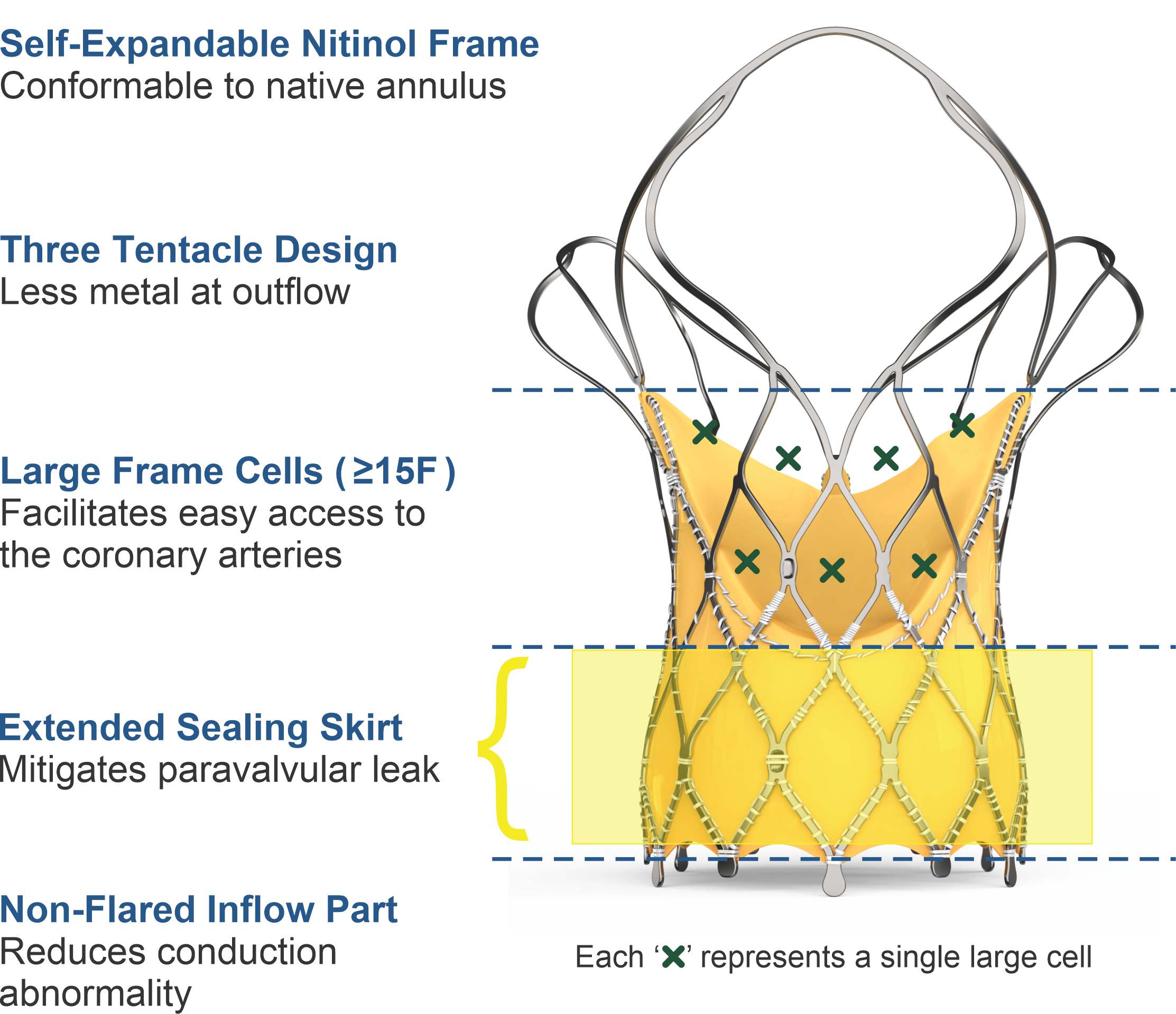
Recapturable
Hydra Aortic Valve
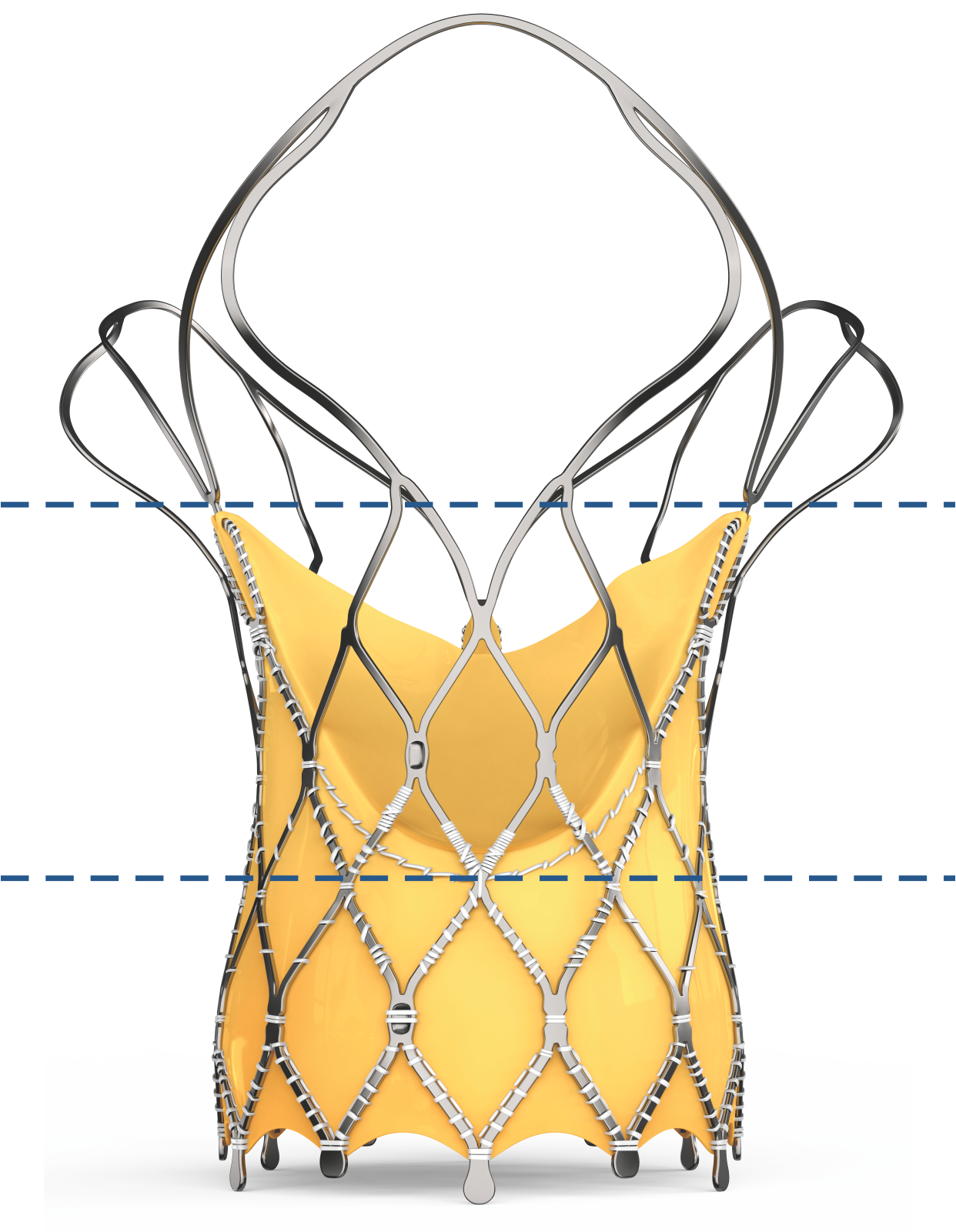
| Outflow | Low radial strength Provides conformability to the shape of the aorta |
| Mid Frame | High hoop strength Ensures circular configuration of the bioprosthetic valve |
| Inflow | High radial strength Provides fixation to the native aortic annulus |
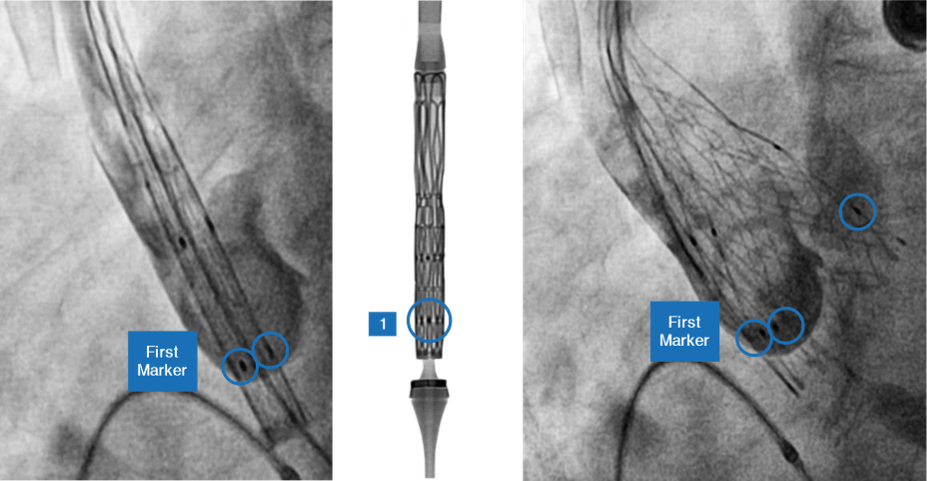
Marker location and significance
- Self-expanding TAVI device to have 2 rows of marker
- First Markers are located at Node 1
- Second Markers are located at Node 3
- First markers help
- In precise implantation of the valve at the targeted implantation zone
- To ascertain the depth of implant
- Second markers indicate
- When the THV leaflets are going to get deployed

Case Example
Hydra AVDC(Aortic Valve Delivery Catheter)
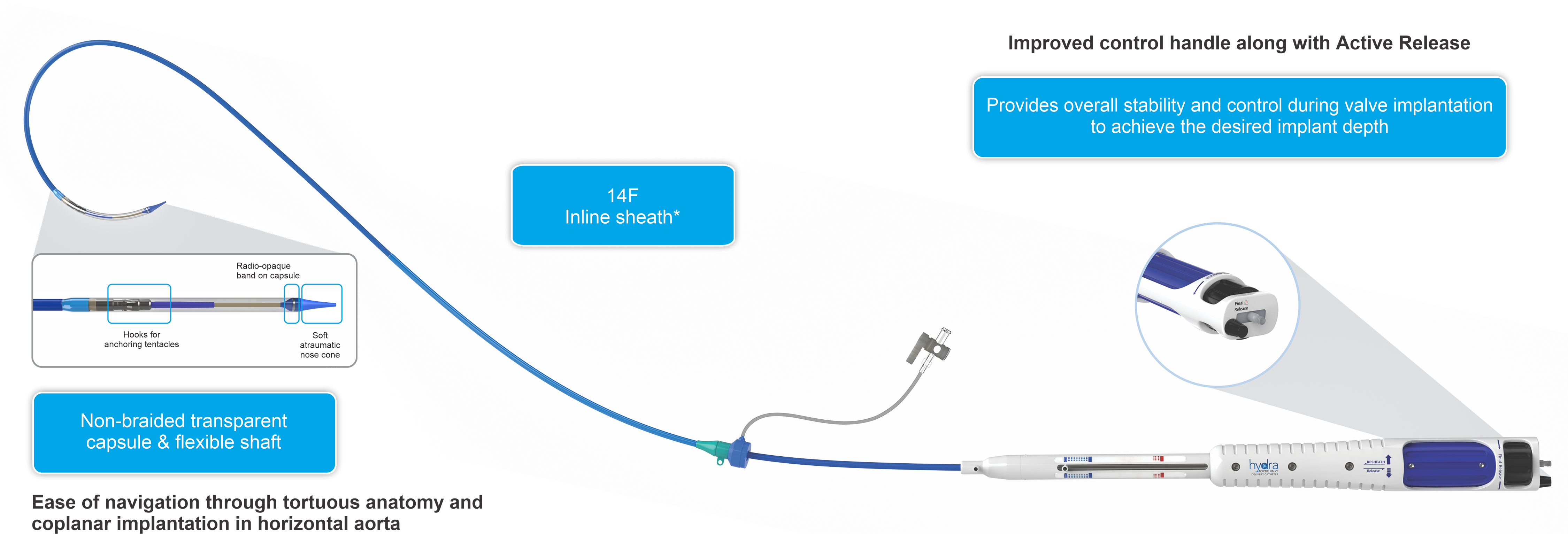
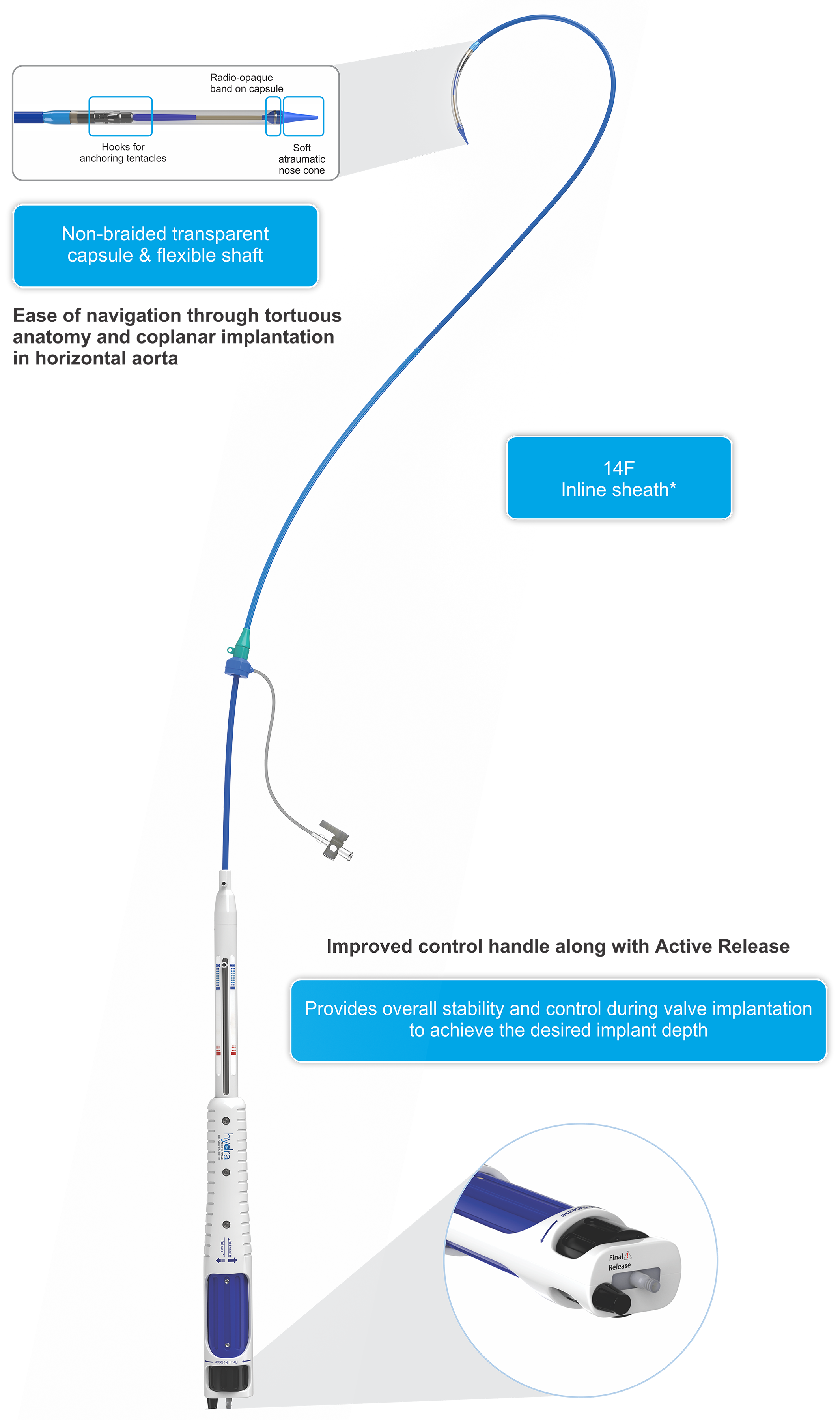
Bovine Pericardium
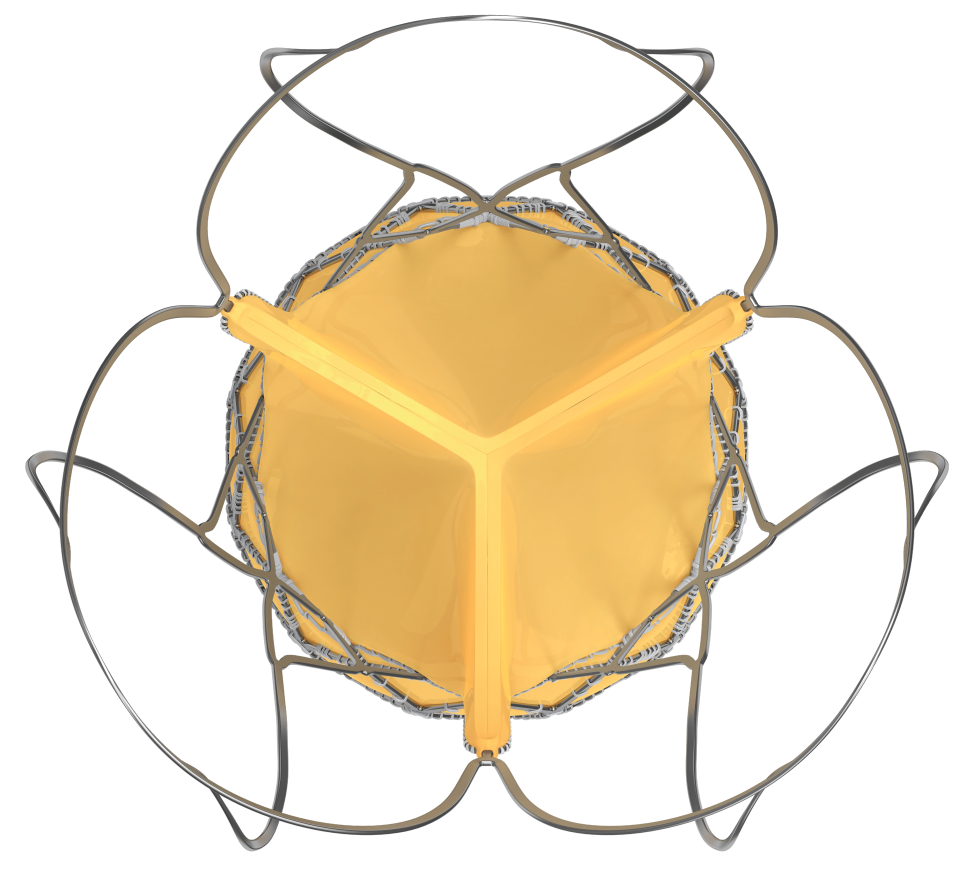
- Made from single bovine pericardium
- Bioprosthetic valve leaflets are supra-annular in position, provide superior hemodynamics by providing larger effective orifice area and lower pressure gradient
- Supra-annular valve position helps to maintain circular shape of the bioprosthetic valve even if the native annulus shape is elliptical
- Proprietary anti-calcification treatment

Clinical experience
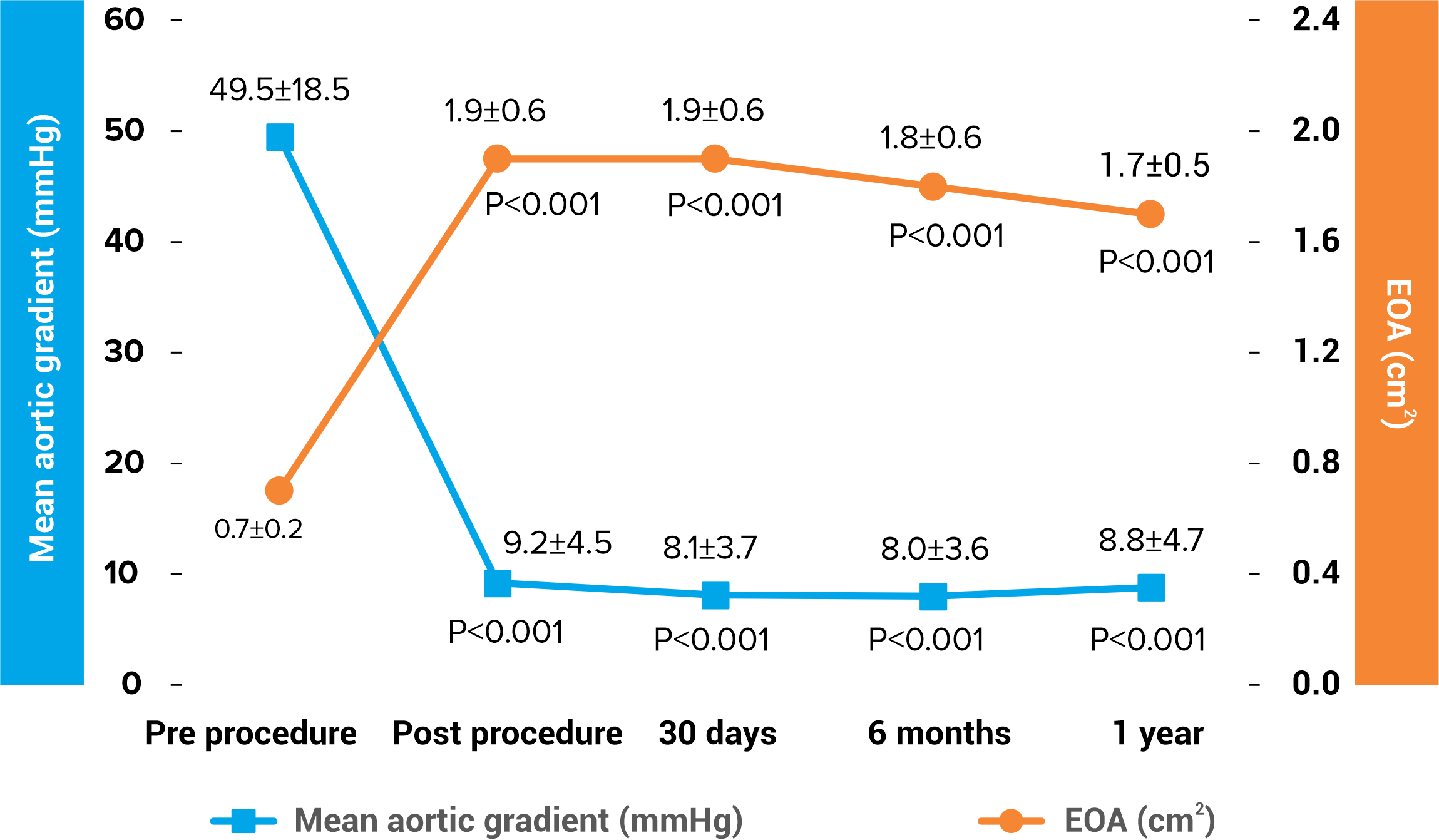
Excellent Hemodynamics
Hydra CE Study (n=157) 1
Single digit valve gradient up to 1-year follow up
Larger effective orifice area (EOA) up to 1-year follow up
Hydra CE Study (n=157) 1
Single digit valve gradient up to 1-year follow up
Larger effective orifice area (EOA) up to 1-year follow up
1. JACC:Cardiovascular Interventions Vol. 15, No. 1, 2022 Jan 10, https://doi.org/10.1016/j.jcin.2021.09.004
Ordering Information
| Reference Number |
Size |
|---|---|
| HYDRA22 | 22 mm |
| HYDRA26 | 26 mm |
| HYDRA30 | 30 mm |
References:
1.Hydra CE study- DOI:10.1016/J.JCIN.2021.09.004
Disclaimers: This product is intended for use by or under the directions of a physician. Prior to use, refer to the “Instructions for use” supplied with the product for indications, contraindications, side effects, suggested procedure warnings and precautions. As part of our continuous product development policy we reserve the right to change product specifications without prior notification.
The Hydra CE study data included here is based on the data published in JACC: Cardiovascular Interventions. JACC: Cardiovascular Interventions is a specialty journal of Journal of the American College of Cardiology (JACC) and is a copyright of ©2022 American College of Cardiology Foundation. JACC: Cardiovascular Interventions encompasses the entire field of interventional cardiovascular medicine, including coronary, structural, peripheral and cerebrovascular interventions.
Caution: Check the regulatory status of the device before distribution in area where CE marking is not the regulation in force. Illustrations are artist’s representation only and should not be considered as engineering drawings or photographs. This information does not replace medical advice from the doctor / physician. Graphics on file at Sahajanand Medical Technologies Limited. Hydra is manufactured by Vascular Innovations Co., Ltd. Thailand, an SMT group company, Hydra is a trademark of Vascular Innovations Co., Ltd. ©2022 Sahajanand Medical Technologies Limited - All Rights Reserved. Specifications are subject to modifications, revision and improvements.


