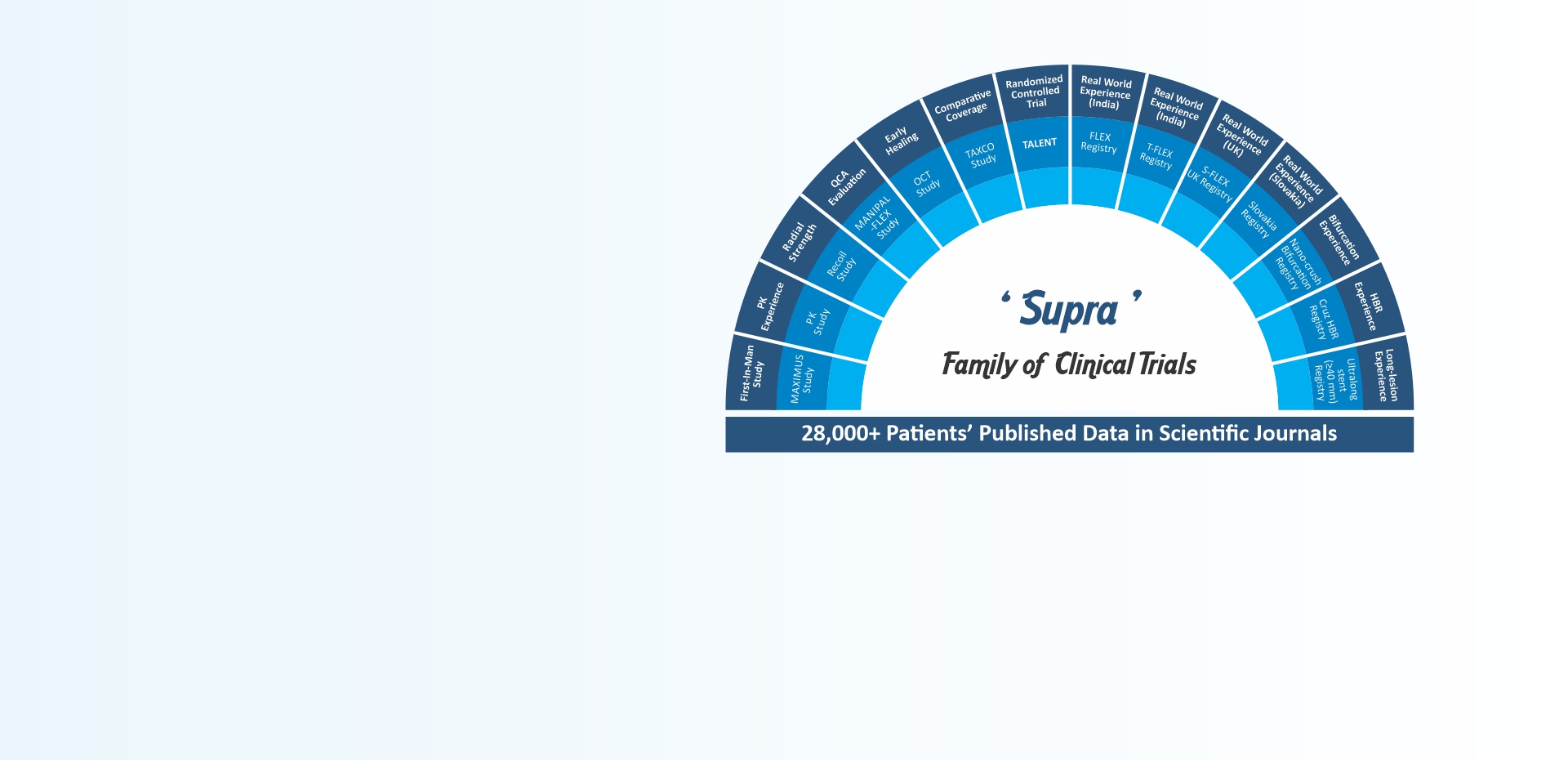
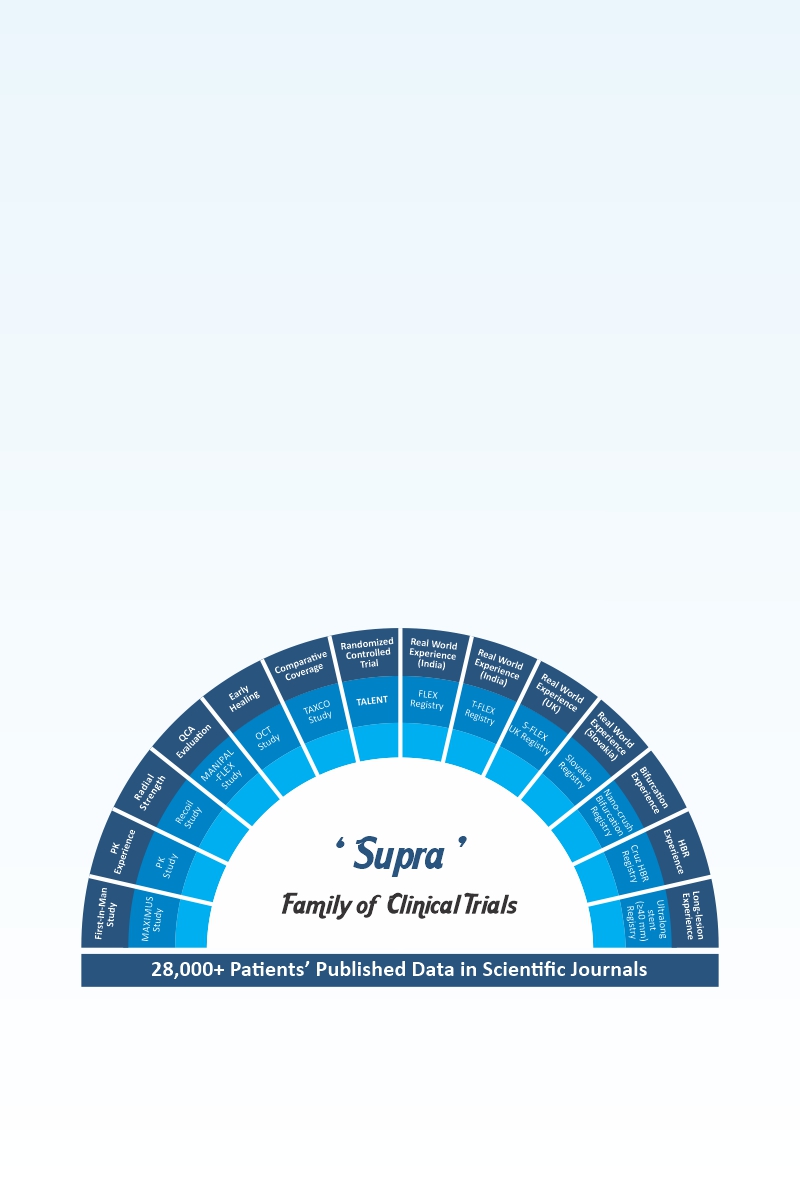
-
Products
-
- Featured Products
- Supraflex Cruz
-
- Professionals
- Patients & Caregivers
- About SMT
Main navigation
Clinical Outcomes of Biodegradable Polymer-coated Ultrathin Strut Sirolimus-eluting Stents in a Real-world Patient Population: T-Flex Registry Three-year Results with High-Risk Subgroups
Prospective Evaluation of Ultrathin-strut Biodegradable Polymer-coated Sirolimus-eluting Stents in an All-comers Patient Population: Slovakia Registry with a Subgroup Analysis
Evaluation of Novel Ultrathin, Biodegradable Polymer-Coated Tetriflex Sirolimus-Eluting Stent Optimization Using Intravascular Ultrasound (IVUS) in Short Coronary Lesions (<20 mm) vs. Long Coronary Lesions (≥20 mm): Tetriflex IVUS Study
Twelve months clinical outcomes of "Nano-crush technique" for the treatment of bifurcation lesions using ultra-thin (60 μm) sirolimus-eluting coronary stents
Clinical outcomes of ultrathin biodegradable polymer-coated sirolimus-eluting stents in an all-comer population: One-year results from the T-FLEX registry including high-risk subgroups
Prospective evaluation of the Supraflex Family sirolimus-eluting coronary stent system in a ‘real-world’ patient population: Interim analysis from the S-FLEX Netherlands Registry
Ultrathin Sirolimus-eluting Stent in Real-world CAD patients: 12 months Results
Ultrathin (60 μm), ultralong (≥40 mm) sirolimus-eluting stent: study of clinical and safety profiles among real-world patients
Healing and early stent coverage after ultrathin strut biodegradable polymer-coated sirolimus-eluting stent implantation: SiBi optical coherence tomography study
Comparison of neointimal coverage between ultrathin biodegradable polymer-coated sirolimus-eluting stents and durable polymer-coated everolimus-eluting stents: 6 months optical coherence tomography follow-up from the TAXCO study
Twelve months clinical outcomes after implantation of long ultra-thin strut biodegradable polymer-coated sirolimus-eluting stents in atherosclerotic coronary lesions
Performance of ultra-thin strut biodegradable polymer-coated sirolimus-eluting stent in females with coronary artery disease: results of twelve months follow-up
Clinical performance of ultra-thin strut biodegradable polymer-coated sirolimus-eluting stents in young patients with coronary artery disease: results of twelve months outcomes
Evaluation of ultra-thin strut biodegradable polymer-coated sirolimus-eluting stent in patients with small coronary arteries (≤2.5 mm)
Evaluation of clinical outcomes after ultra-thin strut (60 µm) biodegradable polymer-coated sirolimus-eluting stent implantation in patients with acute coronary syndrome
One-year clinical outcomes of ultrathin biodegradable polymer coated sirolimus eluting stents in total occlusion: a multicentre, all-comer analysis
One-year clinical outcomes of ultrathin biodegradable polymer coated sirolimus eluting stents for multivessel treatment
One-year clinical outcomes of ultra-thin strut (60 μm) biodegradable polymer-coated sirolimus-eluting coronary stents in diabetic patients
Twelve-month clinical outcomes of an ultra-thin (60 μm) strut, biodegradable polymer-coated, sirolimus-eluting coronary stent in STEMI patients – A real-world, multi-centre experience
Clinical outcomes of biodegradable polymer-coated ultrathin strut sirolimuseluting stents in a “real-world” patient population: two-year results from the T-flex registry
Physiology-guided revascularization versus optimal medical therapy of nonculprit lesions in elderly patients with myocardial infarction: Rationale and design of the FIRE trial
A randomised controlled trial of the sirolimus-eluting biodegradable polymer ultra-thin Supraflex stent versus the everolimus-eluting biodegradable polymer SYNERGY stent for three-vessel coronary artery disease: rationale and design of the Multivessel TAL
Real-world use of ultrathin-strut biodegradable polymer-coated sirolimus-eluting stents in patients with coronary artery disease: 6-month clinical outcomes
Sirolimus-eluting stents with ultrathin struts versus everolimus-eluting stents for patients undergoing percutaneous coronary intervention: final three-year results of the TALENT trial
Prospective Multicenter Randomized All-Comers Trial to Assess the Safety and Effectiveness of the Ultra-Thin Strut Sirolimus-Eluting Coronary Stent Supraflex: Two-Year Outcomes of the TALENT Trial
Slovakia Registry: Prospective evaluation of ultrathin-strut biodegradable polymer-coated sirolimus-eluting stents in an all-comers patient population
The ultra-thin strut sirolimus-eluting coronary stent: SUPRAFLEX
Twelve-month clinical outcomes of sirolimus-eluting stent in coronary artery disease: An experience in real-world Indian patients
Impact of ultra-long sirolimus-eluting stents on coronary artery lesions: one-year results of real-world FLEX-LONG Study
Safety and efficacy of a sirolimus-eluting coronary stent with ultra-thin strut for treatment of atherosclerotic lesions (TALENT): a prospective multicentre randomised controlled trial
A prospective multicentre randomised all-comers trial to assess the safety and effectiveness of the thin-strut sirolimus-eluting coronary stent SUPRAFLEX: rationale and design of the Thin Strut Sirolimus-eluting Stent in All Comers Population vs Everolimu
Prospective evaluation of an ultrathin strut biodegradable polymer-coated sirolimus-eluting stent: 12 months’ results from the S-FLEX UK registry
Three-year outcomes of biodegradable polymer-coated ultra-thin (60 μm) sirolimus-eluting stents in real-world clinical practice
Clinical outcomes and complications of treatment with Supraflex stent in patients with coronary artery disease: One-year follow-up
Clinical outcomes in 995 unselected real-world patients treated with an ultrathin biodegradable polymer-coated sirolimus-eluting stent: 12-month results from the FLEX Registry
Preliminary evaluation of clinical and angiographic outcomes with biodegradable polymer coated sirolimus-eluting stent in de novo coronary artery disease: Results of the MANIPAL-FLEX Study
Comparison of Clinical Outcomes Following Single versus Multivessel Percutaneous Coronary Intervention Using Biodegradable Polymer Coated Sirolimus-Eluting Stent in an All-comers Patient Population
Ultrathin Everolimus-eluting Stent: 3 Years Results with Subgroup of Long Lesions
Serial evaluation of vascular responses after implantation of everolimus-eluting coronary stent by optical coherence tomography
Safety and clinical performance of biodegradable polymer-coated ultra-thin everolimus-eluting stents in “real-world” patients: A Multicenter Registry (PERFORM-EVER)
A “real-world” multicenter registry evaluating safety and clinical performance of biodegradable polymer-coated ultra-thin everolimus-eluting stents: 3-year results from the PERFORM-EVER registry
Safety and performance of everolimus-eluting stents comprising of biodegradable polymers with ultrathin stent platforms
New-generation ultrathin strut (60 μm) everolimus-eluting stents for coronary artery disease: an all-comer study
Twelve-month comparative analysis of clinical outcomes using biodegradable polymer-coated everolimus-eluting stents versus durable polymer-coated everolimus-eluting stents in all-comers patients
Safety and efficacy of a novel everolimus-eluting stent system in “real-world” patients with coronary artery disease: A report of preliminary outcomes
Comparison of biodegradable polymer (Everoflex) vs. permanent polymer (Xience Pro) coated everolimus-eluting coronary stent systems in all-comer patient population at a tertiary care hospital - One-year outcome study
Safety and efficacy of ultra-thin biodegradable polymer-coated everolimus-eluting stent in real-world patients with coronary artery disease – One-year outcomes of Indian multi-centric ‘EVERsafe’ study
Preliminary clinical outcomes after implantation of newer-generation biodegradable polymer-coated everolimus-eluting stent in “real-world” patients with coronary artery disease
Clinical outcomes of successful sirolimus-eluting stent implantation in patients with chronic total occlusion in the real clinical practice
Safety and efficacy of ultra-thin, biodegradable polymer coated sirolimus-eluting Supralimus-core stents in real-world patients: Outcomes at 24-month follow-up
Seven-year clinical outcomes in patients undergoing percutaneous coronary intervention with biodegradable polymer coated sirolimus-eluting stent: Results from a single-center real-world experience
Long-term clinical performance of biodegradable polymer-coated sirolimus-eluting stent in unselected real-world Saudi patients - Seven-year results from multicentre SCORES registry
Long-term clinical outcomes following ultrathin, biodegradable polymer-coated sirolimus-eluting stent implantation in diabetic vs. non-diabetic patients: results from the SCODA registry
Clinical performance of biodegradable polymer-coated sirolimus-eluting stents in unselected real-world population with coronary artery disease: results from the multicenter CORE Registry
Clinical performance of biodegradable polymer coated Sirolimus-eluting stent in multivessel disease: Results from MULTIDES study
The real world experience of the biodegradable polymer-coated sirolimus-eluting coronary stent system: Results From an "All-Comers" Clinical Experience
Evaluation of clinical outcomes in patients undergoing dual vessel percutaneous coronary intervention using sirolimus-eluting coronary stent system in India
Clinical outcomes from unselected “real-world” patients with long coronary lesion receiving 40 mm biodegradable polymer coated sirolimus-eluting stent
Nine-months clinical outcome of biodegradable polymer coated sirolimus-eluting stent system: A multi-Centre “Real-world” experience
Evaluation of prolonged safety and efficacy of biodegradable polymer coated sirolimus-eluting coronary stent system: 1-year outcomes of the INDOLIMUS Registry
Favorable Outcomes after Implantation of Biodegradable Polymer Coated Sirolimus-Eluting Stents in Diabetic Population: Results from INDOLIMUS-G Diabetic Registry
Clinical performance of the cobalt-chromium biodegradable polymer coated sirolimus-eluting stent in an unselected real-world population: S-CORE Registry
Safety and Efficacy of Sirolimus-Eluting Stent in Diabetic Patients Compared with Non-Diabetic Patients Undergoing Percutaneous Coronary Intervention
Very late outcomes of drug-eluting stents coated with biodegradable polymers: insights from the 5-year follow-up of the randomized PAINT trial
Comparison of two biodegradable polymer coated, drug-eluting coronary stents paclitaxel vs. sirolimus, with 6-years clinical follow-up: BIOPRESS(BIOdegradable Polymer Registry Smt Stents) Infinnium vs. Supralimus
Early vascular healing with biodegradable polymer coated sirolimus-eluting coronary stent implantation: assessed by optical coherence tomography results at 4-month follow-up
Clinical Outcomes from Unselected Real-World Patients with Acute Myocardial Infarction Receiving Biodegradable Polymer Coated Sirolimus-Eluting Stents
Experience with biodegradable polymer coated sirolimus-eluting coronary stent system in “real-life” percutaneous coronary intervention: 24-month data from the Manipal-S Registry
A first-in-man study of sirolimus-eluting, biodegradable polymer coated cobalt chromium stent in real life patients
Systemic exposure of sirolimus after coronary stent implantation in patients with de novo coronary lesions: Supralimus-Core® pharmacokinetic study
In vivo assessment of stent recoil of biodegradable polymer- coated cobalt- chromium sirolimus-eluting coronary stent system
Late clinical outcomes after implantation of drug-eluting stents coated with biodegradable polymers: 3-year follow-up of the PAINT randomised trial
Use of Supralimus drug eluting stent with sirolimus and absorbable polymer in the treatment of acute coronary syndrome patients undergoing percutaneous coronary intervention: E- SERIES Registry
Randomized evaluation of two drug-eluting stents with identical metallic platform and biodegradable polymer but different agents (paclitaxel or sirolimus) compared against bare stents: 1-year results of the PAINT trial
Biodegradable-polymer-based, sirolimus-eluting Supralimus® stent: 6-month angiographic and 30-month clinical follow-up results from the Series I prospective study
Analysis of 12 months clinical outcomes associated with implantation of ultrathin (60 μm) bare metal stent in an unselected real-world population with coronary artery disease
Twelve months clinical outcomes after percutaneous coronary intervention with bare metal stents in unselected real-life patients with coronary artery disease: Results from FLEXUS Study
Commissural alignment with the novel Hydra transcatheter heart valve during aortic valve replacement
30-day and 1-year outcomes with hydra self-expanding transcatheter aortic valve: The HYDRA CE study
Clinical evaluation of the Hydra self-expanding transcatheter aortic valve: 6 month results from the GENESIS trial
First-in-man study of transcatheter aortic valve implantations in aortic stenosis using the Hydra self-expanding bioprosthesis
Transcatheter closure of secundum atrial septal defect using Cocoon Septal Occluder: immediate and long-term results
International experience with the use of Cocoon Septal occluder for closure of atrial septal defects
Cocoon devices for transcatheter closure of atrial septal defect and patent ductus arteriosus in children: Single center experience
Safety and efficiency in transcatheter closure of atrial septal defect (ASD) using Cocoon Septal Occluder
Efficacy and safety of catheter closure of atrial septal defects using the Amplatzer versus the Cocoon Septal Occluder. A Multicenter Randomized Study
Long-term safety and efficacy of closure of atrial septal defects with Cocoon Septal Occluder
Transcatheter closure of secundum ASD with Cocoon Septal Occluder in children; early and intermediate term results
Catheter closure of atrial septal defects using the Cocoon Septal Occluder: Preliminary results of a European multicenter study
Clinical results of large secundum atrial septal defect closure in adult using percutaneous transcatheter Cocoon Atrial Septal Occluder
Transcatheter Closure of Patent Foramen Ovale Using the Cocoon Occluder: A Multicenter Retrospective Study
Patent foramen ovale occlusion with the Cocoon PFO Occluder. The PROS-IT collaborative project
New PFO device for closure of patent foramen ovale in patients who had a history of cryptogenic stroke; a report of 14 cases
Cocoon devices for transcatheter closure of atrial septal defect and patent ductus arteriosus in children: Single center experience
Prospective evaluation of the feasibility, safety, and efficacy of Cocoon Duct Occluder for transcatheter closure of large patent ductus arteriosus: A single-center study with short- and medium-term follow-up results
Safety and feasibility of transcatheter interruption of ruptured sinus of valsalva aneurysm using the Cocoon Duct Occluder: Immediate results and mid-term follow-up
Trans catheter closure of perimembranous ventricular septal defects with Cocoon Ductal Occluder devices
Technical considerations for ventricular septal defect device closure using cocoon occluders at tertiary care center in Pakistan
Transcatheter trans-aortic retrograde approach for the closure of perimembranous ventricular septal defects using Cocoon [Amplatzer Duct Occluder I like] device – An initial experience from a single centre
Early experiences using Cocoon Occluders for closure of a ventricular septal defect
Preliminary report of serum nickel concentration after atrial septal defect occlusion with the Cocoon™ device
Transcatheter closure of patent ductus arteriosus with a self-expanding platinum-coated nitinol device
Self-expanding platinum-coated nitinol devices for transcatheter closure of atrial septal defect: Prevention of nickel release